On IVF - A simple introduction
Hello 👋 and welcome to AthenaDAO’s Newsletter where we explore women’s health at the intersection of research, tech, and web3. 🪩
Millions of women every year struggle with infertility. In the United States, 1 in 5 women aged 15 to 49 with no prior births are unable to get pregnant after one year of trying.
Every year, millions of women look for a solution.
From holistic to pharmacological to more invasive approaches, several techniques exist for dealing with infertility. When most holistic (ex. fertility awareness methods) and more conservative treatments such as oral fertility medications with or without intrauterine insemination of sperm don’t work, one technique remains dominant: in vitro fertilization (IVF).

Let’s take a step back… Exactly what is IVF?
by Victoria Dmitruczyk
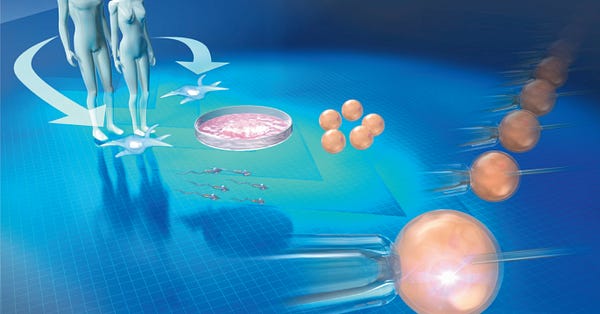
To summarize it very quickly, IVF is the process of stimulating the ovaries to mature several eggs at a time, extracting those eggs outside the body, fertilizing them with sperm outside of the body, and strategically implanting usually one embryo into the uterus to establish a pregnancy.
For this article, we talked extensively with Dr. Paula Amato, a professor of Obstetrics & Gynecology at Oregon Health & Science University, a reproductive endocrinologist, and infertility specialist to ensure our information was correct and clear. Throughout the article, her words, which have been edited for conciseness and clarity, will be referenced.
Pre-Day Zero: Preparation
Before any surgically based procedures can occur, the individual undergoing treatment must prepare their body. Fertility medications are typically taken for several days in each cycle so the ovaries produce mature eggs that can be used in the IVF process.
For some women, orally-based birth control is used prior to starting any treatment to suppress the ovaries to ensure even growth of the eggs and follicles.
*A follicle is a small cystic structure containing fluid and an egg. These are located in the ovaries and there are typically thousands of follicles.
According to Dr. Amato, the goal of ovarian stimulation is to increase the number of mature eggs a patient is able to produce in one cycle. In a typical cycle, only one mature egg is released each month. In an IVF cycle, around 5-15 eggs are induced to grow. When it comes to the number of eggs produced, the biggest factor is age; younger individuals tend to have more follicles and produce more eggs.
Ovarian stimulation occurs by injecting gonadotropins (follicle-stimulating hormone) under the skin. This enhances the growth of the follicles. When the follicles have grown to an appropriate size, hCG (Human Chorionic Gonadotropin - the pregnancy hormone) is administered in order to trigger the follicles into the final stage of maturation.
Dr. Amato: “In a natural cycle, the body releases a surge of hormones called LH prior to ovulation. When women are taking ovulation tests to predict when they are most fertile, this is the hormone that is being detected in the urine. In an IVF cycle, we use a hormone called hCG, which is structurally extremely similar to LH, to trigger the final phase of egg maturation. This is usually given 34-36 hours before egg retrieval.”
Day 0: Fertilization
Once the eggs are mature, in order to retrieve the eggs before they are released from the follicles, a surgical procedure must be performed.
The process is performed under sedation given intravenously. A physician places a thin needle through the vagina into the ovaries under ultrasound guidance to aspirate the follicles using suction, extracting the eggs.
The eggs are taken into a lab where they are fertilized by sperm. There are two ways in which this is done:
Several hundred thousand sperm are placed in a petri dish with one egg, enabling fertilization to occur naturally.
In cases of male infertility or abnormal sperm parameters, one sperm is mechanically injected into each egg, facilitating fertilization.
Days 1-3: Checking for fertilization
The next day, a fertilization check is performed to see how many eggs are fertilized. Embryos are then cultured in the petri dish for 5-6 days.
Days 3-5: Implantation
At this stage, the embryos are ready to be placed into the uterus, a process known as embryo transfer. A doctor will slide a small catheter containing the embryo through the cervix into the uterus and place the embryo in manually and directly under ultrasound guidance. The remaining embryos can be frozen for future use.
Post Day 5: Pregnancy
Once an embryo attaches to the lining of the uterus, pregnancy occurs.
During this phase of the process, progesterone and estrogen are administered for the following 8-10 weeks in order to support the pregnancy.
Unfortunately, not all IVF rounds are successful, and individuals may have to undergo additional cycles before pregnancy success occurs. There are a variety of factors that can impact IVF success rates.
IVF: Will you be successful?
IVF does not guarantee success. On average, each cycle has a less than 50% chance of success. That being said, several things may impact the chance of your success:
In general, as far as success goes, age is the biggest factor. Generally, as women age, egg number and quality decrease, worsening the chances of success with IVF.
AMH (anti-Mullerian hormone) and ovarian follicle count are good markers of egg number. These tests can be ordered by your physician and the hormone levels can be obtained by ordering a kit to do it yourself via mail!
A semen analysis to assess sperm count, number, and morphology (shape) is performed to determine if there is a male factor contributing to infertility
Although IVF is very safe, every medical procedure carries some risks these may include a small risk of OHSS (ovarian hyperstimulation syndrome) and a small risk of bleeding, infection or internal organ injury during the egg retrieval process:
Ovarian Hyperstimulation Syndrome: This occurs when fertility drugs (ex. hCG) cause the ovaries to become overstimulated and swell up releasing excess fluid into the abdomen. This is the most serious complication and it is very rare because it is easy to identify and mitigate the risk.
This cannot happen outside of fertility-related procedures and it is known to be specific to IVF. That being said, if you are taking IVF medications and are undergoing a procedure like intrauterine insemination (IUI), you may be at (very low) risk for this as well.
Other Complications: Stress, nausea, bloating, cramping, tenderness, etc…
IVF pregnancies can be more complicated than spontaneous pregnancies.
Premature delivery: Certain research has suggested that IVF may slightly increase the risk of an early birth when compared to a typical pregnancy.
Ectopic Pregnancy: Around 2.1–8.6% of women will have an ectopic pregnancy following IVF. For context, the typical metric for a natural birth is ~2%.
IVF also holds similar risks to what having a natural birth would have; birth defects, miscarriage, etc… The numbers are too similar to make an estimation as to if IVF has any direct impact and more research is needed.
IVF, although it is very helpful for millions of families, does have its own controversies.
Not a perfect solution for everyone - The accessibility problem.
When it comes to talking about IVF, one of the first things that get mentioned is the cost. In the United States, cost varies, but one IVF cycle can range from around $15k to $40k.
When talking to Dr. Melissa Walsh, an OB/GYN physician and web3 healthcare advocate, she stated: “This is the cost for one cycle! If the procedure doesn’t work, the individual(s) undergoing the process would have to pay this out again. There are severe barriers in terms of accessibility due to these costs and the groups we often see as underprivileged in society also face a lack of access to such treatments.”
Outside of the United States, where healthcare is covered or better subsidized, IVF is more financially accessible. However, the non-upfront costs (such as taking time off of work to deal with necessary office visits) are still not accounted for. Additionally, women are criticized for talking about their symptoms and struggles in health, although this is a much wider issue for the entire spectrum of women’s health. There are other factors to consider in regard to accessibility. For instance, certain cultures have religious obligations to IVF.
A look around the world:
What could the future of IVF look like if we invested in more research?
When talking to Dr. Amato, she mentioned 3 key things:
Accessibility. Ideally, in the future, IVF would become a less strenuous process, accessible to more people and multiple different populations in all countries (ex. LGBTQ) for a lower cost.
More successful. In the future, as IVF research continues, it is likely that the success rates will improve. This can come from figuring out ways to have better quality eggs, sperm, embryos, and better implantation rates.
Better involvement with emerging tech. For example, at her lab, she’s trying to understand how to turn skin cells into egg cells. There are lots of other emerging ideas that will likely have some sort of influence in the IVF field. For instance, maturing egg in a petri dish instead of in the body (a process called in vitro maturation of eggs),
Dr. Walsh believes a future with more research investment in women’s health and fertility combined with AI/ML data analytics will improve current IVF treatments and advance our understanding of the body, menstrual cycle, and ovulatory factors which can influence our fertility among other areas. She states that AthenaDAO is poised to make women’s reproductive health part of the main conversation by transforming the research process and growing educational awareness through its decentralized community of advocates.
We’ll be hosting a Twitter Spaces panel with Dr. Melissa Walsh on the topic of IVF on Thursday, February 9th, 2023 at 9 am PST / 12 pm EST / 6 pm CET.
Save the date HERE.
If you have any questions about this article or want to know more about the topic of IVF, we’d love for you to join!
The future of IVF
Dr. Paula Amato has been sharing some of the most relevant and interesting news around fertility and IVF on her Twitter, you should follow it.
One that struck a chord in relation to Decentralized Science was an article on “Intellectual property and assisted reproductive technology”
The evolution of IVF is IVG (in vitro gametogenesis), the production of eggs and sperm from undifferentiated human cells.
“It is likely that IVG will fundamentally change how humans reproduce. IVG could offer the possibility of reproduction to those experiencing infertility, allow parents to choose from hundreds of genetically characterized embryos, enable relatively safe germline genetic modification, and open the door for same-sex parents to have genetically related offspring.”
Whether or not you are excited about these advancements, we must start questioning who will own the technologies that researchers create. IVF was largely unencumbered by patents, but with IVG, a number of companies have already started filing various patents to claim ownership of techniques.
How do we balance incentive versus access?
“IVG could set a new standard of reproductive health, with social implications far beyond its predecessor, IVF. But unlike IVF, the administration of IVG may shift from thousands of independent clinics around the world to a handful of corporate providers or their licensees.”
Do we want this to be accessible only to those who can afford it? Should reproductive technology be monopolized?
DeSci and IVF - IP Innovation
We do think that IP-NFTs owned by communities could play an important role in making sure that academic research and its discoveries are more broadly distributed.
Anyone who’s followed AthenaDAO would know by now, women’s health, especially reproductive health, is an untapped opportunity.
We do think that IP-NFTs owned by collectives such as AthenaDAO, where patients and advocates also have a say, could play an important role in making sure that academic research and the fruits of it are more broadly distributed, and are not fully monopolizable.
Most researchers face barriers to funding. Dr. Amato stated that the NIH does not fund human embryo research and that the FDA doesn’t like to consider human trials when it comes to genetic modification of human embryos.
When they do get funding, sometimes, they are not the ones getting the most benefits from such IP either - that’s another conversation on its own.
There is a more interesting proposition; Decentralized Science brings a new proposition to IP: the notion that researchers deserve to have intellectual property in their own research and that this science should lead to more public goods.

References:




